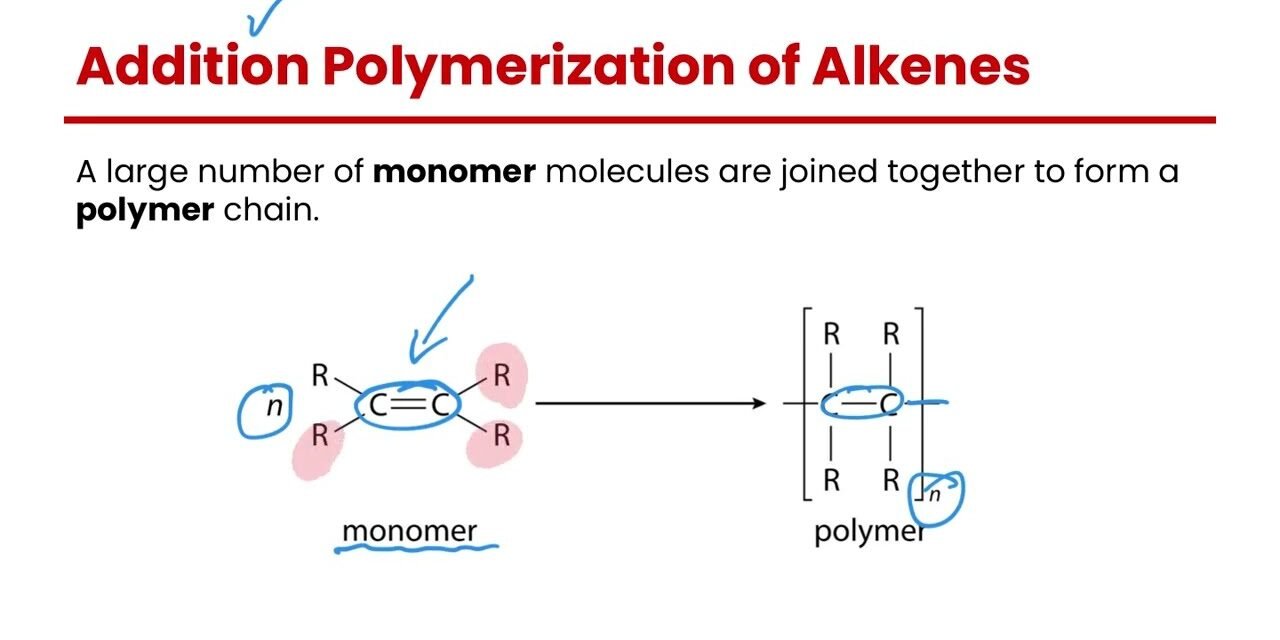
Polymerization is the process through which monomers (small molecules) chemically bond to form polymers (large molecules with repeating units). There are several methods of polymerization, each affecting the properties of the resulting polymers. The two most common types of polymerization are addition polymerization and condensation polymerization. Below, I’ll explain these methods in detail, how they work, and how they influence the properties of the resulting polymers.
1. Addition Polymerization (Chain-Growth Polymerization)
How It Works:
- Initiation: The polymerization begins with the generation of free radicals, cationic, or anionic species that can react with monomers containing double bonds (typically alkenes). This creates a reactive site on the monomer, starting the chain reaction.
- Propagation: The reactive species (usually a free radical) adds to the double bond of a monomer, opening up its bond and allowing more monomers to add, forming a growing polymer chain.
- Termination: The polymerization ends when two growing chains combine, or when a terminator is introduced to stop the chain reaction.
Types of Addition Polymerization:
- Free Radical Polymerization: The most common method, used to produce polyethylene (PE), polystyrene (PS), and polyvinyl chloride (PVC).
- Cationic Polymerization: Involves the use of positively charged species to initiate polymerization (e.g., polymethyl methacrylate (PMMA)).
- Anionic Polymerization: Uses negatively charged species to initiate polymerization (e.g., polybutadiene).
Polymer Properties Influenced by Addition Polymerization:
- Molecular Weight: The polymer chain length is often controlled during polymerization to affect tensile strength, viscosity, and other material properties.
- Degree of Polymerization: This determines how long the polymer chains are, which impacts the polymer’s mechanical properties such as toughness and elasticity.
- Tacticity: The spatial arrangement of side groups on the polymer chain affects the polymer’s crystallinity and thermal properties. For example, isotactic (uniform) polymers tend to be more crystalline and stronger than atactic (random) polymers.
Examples of Polymers Made by Addition Polymerization:
- Polyethylene (PE): Commonly used in plastic bags, bottles, and pipes. The polymerization process allows for varying densities of polyethylene (HDPE, LDPE, LLDPE), which influences their strength, flexibility, and chemical resistance.
- Polystyrene (PS): A rigid, clear plastic used in packaging, disposable cutlery, and insulation.
2. Condensation Polymerization (Step-Growth Polymerization)
How It Works:
- Reaction between Monomers: Condensation polymerization involves two or more monomers, each with at least two reactive groups (e.g., hydroxyl, amine, or carboxyl groups). When these groups react, they form a covalent bond, releasing a small by-product such as water or methanol.
- Step-Growth: Unlike addition polymerization, the monomers can react with each other at any stage, so the polymer grows by forming bonds between any two reactive species (not just at the ends of chains). This typically leads to a more complex, step-growth polymerization process.
Types of Condensation Polymerization:
- Polyester Polymerization: Commonly used to produce polyethylene terephthalate (PET), which is used in plastic bottles, clothing fibers, and films.
- Polyamide Polymerization: Used to create nylon (e.g., Nylon-6,6), which is used in textiles, automotive parts, and industrial applications.
- Urethane Polymerization: Produces polyurethanes used in foams, coatings, and adhesives.
Polymer Properties Influenced by Condensation Polymerization:
- High Molecular Weight: Condensation polymerization typically results in polymers with high molecular weights, which often enhances mechanical strength, chemical resistance, and thermal stability.
- Structural Features: Because the polymer chains are connected through functional groups (such as amide, ester, or urethane groups), the resulting polymers often have high crystallinity and rigidity, depending on their monomer structure.
- Thermal Properties: Polymers like polyethylene terephthalate (PET) and nylon exhibit excellent heat resistance and strength, making them suitable for demanding applications like automotive parts and engineering plastics.
Examples of Polymers Made by Condensation Polymerization:
- Polyethylene Terephthalate (PET): Commonly used in plastic bottles and food packaging. The molecular weight and degree of crystallinity can be controlled to optimize its properties for strength, clarity, and durability.
- Nylon (Polyamide): A strong, abrasion-resistant polymer used in fibers, ropes, and automotive components. Its properties are influenced by the length of the polymer chains and the degree of crystallinity.
- Polyurethanes (PU): Used in foams, coatings, adhesives, and sealants. The type of monomer used (diols, diisocyanates) and the degree of crosslinking significantly influence the flexibility and hardness of the material.
3. Influence of Polymerization Type on Properties
- Addition Polymerization: Polymers made through addition polymerization tend to have high molecular weight, flexibility, and good impact resistance. However, they may not perform well in high-temperature environments or under stress for long periods, unless specially modified (e.g., through crosslinking).
- Example: Polypropylene is a versatile thermoplastic with good chemical resistance and flexibility, but it lacks the high-temperature resistance of thermosets.
- Condensation Polymerization: Polymers formed via condensation polymerization typically exhibit higher strength, rigidity, and thermal stability because of the crosslinking and functional group interactions. These polymers are often more durable and resistant to heat and chemicals than those made via addition polymerization.
- Example: Nylon and PET are known for their high mechanical strength, abrasion resistance, and chemical resistance, making them suitable for more demanding applications in engineering and automotive industries.
Conclusion
The type of polymerization used—addition or condensation—significantly influences the properties of the resulting polymers, including their molecular weight, strength, flexibility, and thermal stability. Addition polymerization is ideal for mass production of polymers like PE, PP, and PS, where ease of processing and recyclability are key advantages. On the other hand, condensation polymerization is suited for high-performance polymers like PET, nylon, and polyurethane, which are used in automotive, aerospace, and engineering applications requiring high strength, heat resistance, and chemical stability. Understanding these processes helps manufacturers select the appropriate polymerization method for their specific application needs.
Hashtags
#Polymerization #AdditionPolymerization #CondensationPolymerization #PolymerChemistry #PolymerProperties #PolymerizationMethods #PolymerizationTypes #PlasticProperties #MolecularWeight #PolymerStructure #PolymerizationTechniques #MaterialScience #SustainablePolymers #PolymerizationProcess #PlasticManufacturing #Chemistry #Innovation