Monomers are the building blocks of polymers, and their sourcing is integral to the production of various polymer materials used in the plastics and rubber industries. The most common monomers used in polymer manufacturing are derived from petrochemicals, although there is also a growing interest in bio-based monomers. Here’s a detailed look at how monomers are sourced and the role petrochemicals play in polymer manufacturing:
1. Sourcing of Monomers
Monomers can be sourced from different natural and synthetic processes, but the majority of commercially used monomers are derived from petrochemical sources. Here’s an overview of the sources:
a) Petrochemical Sourcing (Fossil Fuels)
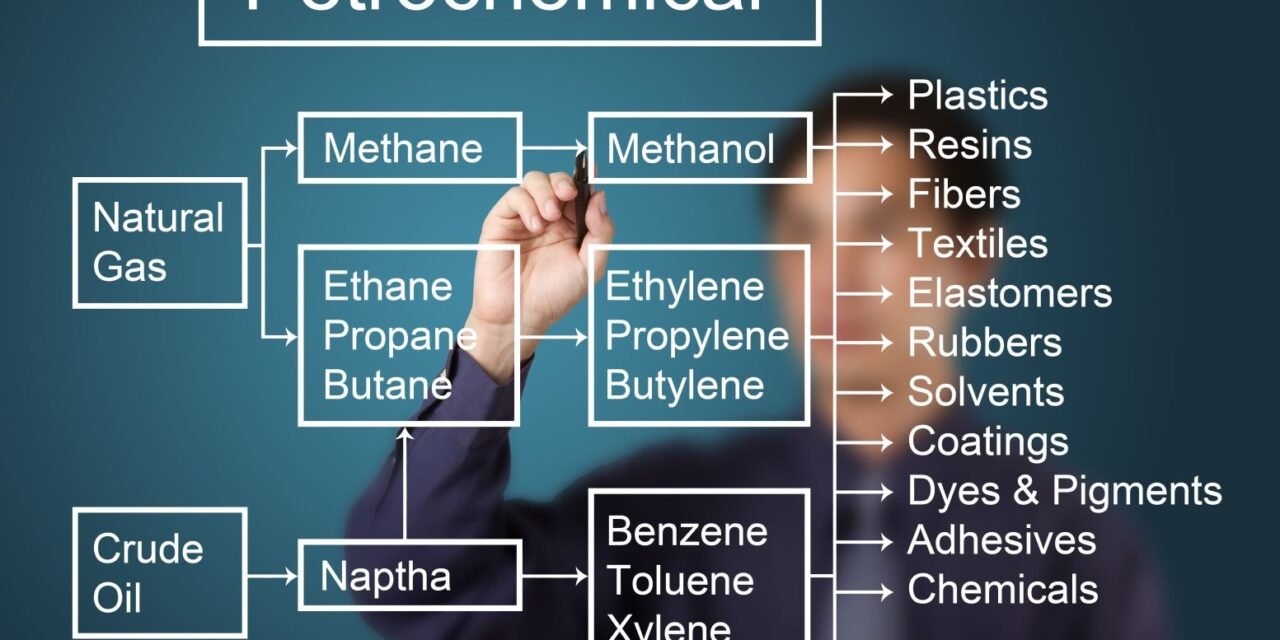
- Crude Oil and Natural Gas: Most petrochemical monomers are derived from crude oil and natural gas through a series of chemical processes. These fossil fuels serve as feedstocks for the production of a wide range of monomers used in plastics and rubber manufacturing.
- Key Petrochemical Processes:
- Cracking: Involves breaking down larger hydrocarbons into smaller molecules. Ethylene, propylene, and butadiene are commonly produced from cracking of hydrocarbons in crude oil and natural gas.
- Reforming: A process where hydrocarbons are rearranged to produce desired monomers. This is used to produce aromatic compounds such as benzene, toluene, and xylene.
- Polymerization: Once monomers are produced, they undergo polymerization processes (e.g., addition polymerization or condensation polymerization) to form polymers.
- Common Petrochemical Monomers:
- Ethylene (C2H4): Derived from cracking naphtha or natural gas, ethylene is a key monomer for making polyethylene (PE), ethylene-vinyl acetate (EVA), and polystyrene (PS).
- Propylene (C3H6): Sourced from crude oil and natural gas, propylene is used to produce polypropylene (PP) and acrylonitrile-butadiene-styrene (ABS).
- Butadiene (C4H6): Produced from cracking and used to make styrene-butadiene rubber (SBR) and butadiene rubber.
- Benzene, Toluene, Xylene: Aromatic compounds derived from petroleum used in the production of styrene and nylon.
b) Bio-Based Monomers (Renewable Resources)
- Bio-based feedstocks are increasingly being used to produce bio-based monomers as part of a push for sustainable materials and reducing reliance on fossil fuels.
- Bio-Based Monomers Examples:
- Lactic Acid: Used to make polylactic acid (PLA), a biodegradable plastic derived from corn starch or sugarcane.
- Succinic Acid: A bio-based monomer that can be used to produce bio-based polyesters and polyurethanes.
- 1,3-Propanediol: Sourced from glucose or glycerol and used in the production of bio-based polytrimethylene terephthalate (PTT).
- Isosorbide: Derived from renewable feedstocks such as corn, it can replace petroleum-based monomers in making bio-based polymers.
c) Synthesis from Biomass and Waste:
- Biomass and waste materials (such as agricultural residues or even carbon dioxide) are being explored as alternative sources for producing monomers. This approach aims to reduce carbon footprints and support circular economies.
2. Role of Petrochemicals in Polymer Manufacturing
Petrochemicals play a central role in polymer manufacturing because they provide the essential monomers that serve as the foundation for producing a wide array of plastics and rubber. Here’s how petrochemicals are used in polymer manufacturing:
a) Production of Key Monomers
- Ethylene: Produced primarily through the cracking of naphtha and natural gas liquids (NGLs), ethylene is one of the most widely used monomers. It forms the basis for polyethylene (PE), one of the most commonly used plastics.
- Propylene: Produced alongside ethylene, propylene is used in the production of polypropylene (PP), another widely used polymer in packaging, automotive, and textiles.
- Styrene: Derived from benzene and ethylene, styrene is a key monomer for the production of polystyrene (PS) and styrene-butadiene rubber (SBR).
- Butadiene: Produced from crude oil through the cracking process, butadiene is used in the production of synthetic rubbers like SBR and polybutadiene.
- Aromatic Compounds: Benzene, toluene, and xylene are key monomers for producing various engineering plastics like polycarbonate (PC), polyurethane (PU), and nylon.
b) Polymerization to Form Plastics
- Once monomers are sourced, they undergo polymerization to form long-chain polymers. In addition polymerization, ethylene produces polyethylene (PE), propylene produces polypropylene (PP), and styrene produces polystyrene (PS).
- Condensation polymerization using benzene or terephthalic acid produces polyesters (e.g., polyethylene terephthalate (PET)), which are used in textiles, packaging, and bottles.
c) Enhancing Polymer Properties
- Petrochemicals can also be used to modify polymers, improving chemical resistance, thermal stability, and flexibility. Additives like plasticizers (for PVC), reinforcements (e.g., glass fibers), and stabilizers (e.g., UV stabilizers) can be incorporated into the polymer to enhance its properties for specific applications.
- For example, polypropylene (PP) is often blended with additives to make it more impact-resistant or to improve its optical properties.
3. Environmental Impact and Alternatives
The reliance on petrochemicals in polymer production raises environmental concerns due to their non-renewable nature, the carbon emissions associated with fossil fuel extraction and processing, and the pollution linked to plastic waste. To mitigate these issues, there are efforts to:
a) Promote Bio-based Alternatives:
- Bio-based monomers made from renewable resources, such as PLA (from corn starch) and PHA (from bacterial fermentation), are gaining traction as sustainable alternatives to petrochemical-based polymers. These bio-based materials are more biodegradable and reduce the carbon footprint of plastics.
b) Improve Recycling and Circular Economy:
- Recycling technologies, such as chemical recycling and mechanical recycling, are being developed to recycle petrochemical-based polymers, like polyethylene (PE) and polypropylene (PP), into new materials, reducing the need for new monomer production.
- Additionally, upcycling is being explored to transform waste plastics into higher-value products, further promoting the circular economy.
c) Use of Renewable Energy:
- Shifting the energy sources for petrochemical production to renewable energy (e.g., wind or solar) helps reduce the overall carbon emissions from polymer manufacturing.
Conclusion
Monomers are essential building blocks in polymer manufacturing, and most monomers are traditionally sourced from petrochemicals, derived from crude oil and natural gas. These monomers undergo polymerization to form a variety of plastics and rubbers used in many industries, including automotive, packaging, electronics, and medical devices. While petrochemicals have long been the primary source of monomers, there is a growing shift toward bio-based alternatives and recycling technologies to address environmental concerns related to the reliance on fossil fuels and the non-biodegradability of plastics. As demand for sustainable materials grows, the future of polymer manufacturing may involve a more diverse mix of renewable resources and advanced recycling methods.
Hashtags
#PolymerMonomers #Petrochemicals #MonomerSourcing #PolymerManufacturing #PetrochemicalIndustry #SustainableMonomers #ChemicalEngineering #PlasticProduction #MonomerProduction #FossilFuelsInPlastics #RenewableMonomers #Polymerization #PlasticsFromPetrochemicals #MonomerApplications #SustainablePolymers #GreenChemistry